Metal theme by Jabyaeye
Download: Metal_2.p3t

(5 backgrounds)


| Part of a series on the |
| Periodic table |
|---|
A metal (from Ancient Greek μέταλλον (métallon) 'mine, quarry, metal') is a material that when polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not.[1]: Chpt 8 & 19 [2]: Chpt 7 & 8 Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets).[3]
A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride.[4] The general science of metals is called metallurgy, a subtopic of materials science; aspects of the electronic and thermal properties are also within the scope of condensed matter physics and solid-state chemistry, it is a multidisciplinary topic.
A metal conducts electricity at a temperature of absolute zero,[5] which is a consequence of the states at the Fermi energy.[1][2] Many elements and compounds become metallic under high pressures, for example, iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Sodium becomes a nonmetal at pressure of just under two million times atmospheric pressure, and at even higher pressures it is expected to become a metal again
When discussing the periodic table and some chemical properties the term metal is often used to denote those elements which in pure form and at standard conditions are metals in the sense of electrical conduction mentioned above. The related term metallic may also be used for types of dopant atoms or alloying elements.
In astronomy metal refers to all chemical elements in a star that are heavier than helium. In this sense the first four "metals" collecting in stellar cores through nucleosynthesis are carbon, nitrogen, oxygen, and neon. A star fuses lighter atoms, mostly hydrogen and helium, into heavier atoms over its lifetime. The metallicity of an astronomical object is the proportion of its matter made up of the heavier chemical elements.[6][7]
The strength and resilience of some metals has led to their frequent use in, for example, high-rise building and bridge construction, as well as most vehicles, many home appliances, tools, pipes, and railroad tracks. Precious metals were historically used as coinage, but in the modern era, coinage metals have extended to at least 23 of the chemical elements.[8] There is also extensive use of multi-element metals such as titanium nitride or degenerate semiconductors in the semiconductor industry.
The history of refined metals is thought to begin with the use of copper about 11,000 years ago. Gold, silver, iron (as meteoric iron), lead, and brass were likewise in use before the first known appearance of bronze in the fifth millennium BCE. Subsequent developments include the production of early forms of steel; the discovery of sodium—the first light metal—in 1809; the rise of modern alloy steels; and, since the end of World War II, the development of more sophisticated alloys.
Properties[edit]
Form and structure[edit]

Most metals are shiny and lustrous, at least when polished, or fractured. Sheets of metal thicker than a few micrometres appear opaque, but gold leaf transmits green light. This is due to the electrons which reflect light.[1][2]
Although most elemental metals have higher densities than nonmetals,[9] is a wide variation in their densities, lithium being the least dense (0.534 g/cm3) and osmium (22.59 g/cm3) the most dense. (Some of the 6d transition metals are expected to be denser than osmium, but predictions on their densities vary widely in the literature, and in any case their known isotopes are too unstable for bulk production to be possible.) Magnesium, aluminium and titanium are light metals of significant commercial importance. Their respective densities of 1.7, 2.7, and 4.5 g/cm3 can be compared to those of the older structural metals, like iron at 7.9 and copper at 8.9 g/cm3. An iron ball would thus weigh about as much as three aluminum balls of equal volume.

Metals are typically malleable and ductile, deforming under stress without cleaving.[9] The nondirectional nature of metallic bonding contributes to the ductility of most metallic solids, where the Peierls stress is relatively low allowing for dislocation motion, and there are also many combinations of planes and directions for plastic deformation.[10] Due to their having close packed arrangements of atoms the Burgers vector of the dislocations are fairly small, which also means that the energy needed to produce one is small.[3][10] In contrast, in an ionic compound like table salt the Burgers vectors are much larger and the energy to move a dislocation is far higher.[3] Reversible elastic deformation in metals can be described well by Hooke's Law for the restoring forces, where the stress is linearly proportional to the strain.[11]
A temperature change may lead to the movement of structural defects in the metal such as grain boundaries, point vacancies, line and screw dislocations, stacking faults and twins in both crystalline and non-crystalline metals. Internal slip, creep, and metal fatigue may also ensue.[3][10]
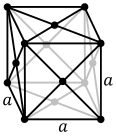
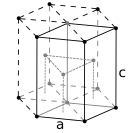
The atoms of simple metallic substances are often in one of three common crystal structures, namely body-centered cubic (bcc), face-centered cubic (fcc), and hexagonal close-packed (hcp). In bcc, each atom is positioned at the center of a cube of eight others. In fcc and hcp, each atom is surrounded by twelve others, but the stacking of the layers differs. Some metals adopt different structures depending on the temperature.[12]
-
Body-centered cubic crystal structure, with a 2-atom unit cell, as found in e.g. chromium, iron, and tungsten
-
Face-centered cubic crystal structure, with a 4-atom unit cell, as found in e.g. aluminum, copper, and gold
-
Hexagonal close-packed crystal structure, with a 6-atom unit cell, as found in e.g. titanium, cobalt, and zinc
Many other metals with different elements have more complicated structures, such as rock-salt structure in titanium nitride or perovskite (structure) in some nickelates.[13]
Electrical and thermal[edit]

The electronic structure of metals means they are relatively good conductors of electricity. The electrons all have different momenta, which average to zero when there is no external voltage. When a voltage is applied some move a little faster in a given direction, some a little slower so there is a nett drift velocity which leads to an electric current.[1][2] Quantum mechanics dictates that one can only have one electron in a given states, the Pauli exclusion principle.[14] Therefore there have to be empty states available at the highest occupied energies as sketched in the Figure. In a semiconductor like silicon or a nonmetal like strontium titanate there is an energy gap between the highest filled states of the electrons and the lowest unfilled. Consequently, semiconductors and nonmetals are relatively poor conductors, although they can carry some current when doped with elements that introduce additional energy states or at higher temperatures.[15]
The elemental metals have electrical conductivity values of from 6.9 × 103 S/cm for manganese to 6.3 × 105 S/cm for silver. In contrast, a semiconducting metalloid such as boron has an electrical conductivity 1.5 × 10−6 S/cm. With one exception, metallic elements reduce their electrical conductivity when heated. Plutonium increases its electrical conductivity when heated in the temperature range of around −175 to +125 °C, with anomalously large thermal expansion coefficient and a phase change from monoclinic to face-centered cubic near 100 °C.[16]
Metals are relatively good conductors of heat. At higher temperatures they can occupy slightly higher energy levels which are given by Fermi–Dirac statistics.[2][15] These have slightly higher momenta (kinetic energy) so can pass on thermal energy.
The contribution of a metal's electrons to its heat capacity and thermal conductivity, and the electrical conductivity of the metal itself can be approximately calculated from the free electron model.[2] However, this does not take into account the detailed structure of the metal's ion lattice. Taking into account the positive potential caused by the arrangement of the ion cores enables consideration of the electronic band structure and binding energy of a metal. Various models are applicable, the simplest being the nearly free electron model.[2] Modern methods such as density functional theory are typically used.
Chemical[edit]
The elements which form metals usually form cations through electron loss.[9] Most will react with oxygen in the air to form oxides over various timescales (potassium burns in seconds while iron rusts over years) which depend upon whether the native oxide forms a passivation layer that acts as a
Comments are closed.
6 Replies to “Metal #2”


Contains 5 original backgrounds. Icons are from The Method
freakn cool man. i think you did a good job!!!
not bad but this had more votes than 2 on friday 19/06/09. must use a reliable system here.
i gave it vote no 3 today but it still says 2 couple of hours later
My vote on this theme 21/06/09(was vote number 3) & Did not take the score to 4.67. Instead of changing number of votes & scores on these themes, Could you upload the next batch of themes before the links run out please.
& now it trys to stop me checking this theme altogether, i like the theme, Though the fake score still confuses me. If its 4.67 why not on home page scores.