TNT theme by TNT
Download: TNT_2.p3t

(1 background)
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methyl-1,3,5-trinitrobenzene[citation needed] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | TNT | ||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.900 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 0209 – Dry or wetted with < 30% water 0388, 0389 – Mixtures with trinitrobenzene, hexanitrostilbene | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H5N3O6 | |||
| Molar mass | 227.132 g·mol−1 | ||
| Appearance | Pale yellow solid. Loose "needles", flakes or prills before melt-casting. A solid block after being poured into a casing. | ||
| Density | 1.654 g/cm3 | ||
| Melting point | 80.35 °C (176.63 °F; 353.50 K) | ||
| Boiling point | 240.0 °C (464.0 °F; 513.1 K) (decomposes)[1] | ||
| 0.13 g/L (20 °C) | |||
| Solubility in ether, acetone, benzene, pyridine | soluble | ||
| Vapor pressure | 0.0002 mmHg (20°C)[2] | ||
| Explosive data | |||
| Shock sensitivity | Insensitive | ||
| Friction sensitivity | Insensitive to 353 N | ||
| Detonation velocity | 6900 m/s | ||
| RE factor | 1.00 | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H201, H301, H311, H331, H373, H411 | |||
| P210, P273, P309+P311, P370+P380, P373, P501 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
795 mg/kg (rat, oral) 660 mg/kg (mouse, oral)[3] | ||
LDLo (lowest published)
|
500 mg/kg (rabbit, oral) 1850 mg/kg (cat, oral)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1.5 mg/m3 [skin][2] | ||
REL (Recommended)
|
TWA 0.5 mg/m3 [skin][2] | ||
IDLH (Immediate danger)
|
500 mg/m3[2] | ||
| Safety data sheet (SDS) | ICSC 0967 | ||
| Related compounds | |||
Related compounds
|
picric acid hexanitrobenzene 2,4-Dinitrotoluene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Trinitrotoluene (/ˌtraɪˌnaɪtroʊˈtɒljuiːn/),[4][5] more commonly known as TNT (and more specifically 2,4,6-trinitrotoluene), and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene,[citation needed] is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.
History[edit]
TNT was first prepared in 1861 by German chemist Joseph Wilbrand[6] and originally used as a yellow dye. Its potential as an explosive was not recognized for three decades, mainly because it was too difficult to detonate because it was less sensitive than alternatives. Its explosive properties were discovered in 1891 by another German chemist, Carl Häussermann.[7] TNT can be safely poured when liquid into shell cases, and is so insensitive that in 1910 it was exempted from the UK's Explosives Act 1875 and was not considered an explosive for the purposes of manufacture and storage.[8]
The German armed forces adopted it as a filling for artillery shells in 1902. TNT-filled armour-piercing shells would explode after they had penetrated the armour of British capital ships, whereas the British Lyddite-filled shells tended to explode upon striking armour, thus expending much of their energy outside the ship.[8] The British started replacing Lyddite with TNT in 1907.[9]
The United States Navy continued filling armour-piercing shells with explosive D after some other nations had switched to TNT, but began filling naval mines, bombs, depth charges, and torpedo warheads with burster charges of crude grade B TNT with the color of brown sugar and requiring an explosive booster charge of granular crystallized grade A TNT for detonation. High-explosive shells were filled with grade A TNT, which became preferred for other uses as industrial chemical capacity became available for removing xylene and similar hydrocarbons from the toluene feedstock and other nitrotoluene isomer byproducts from the nitrating reactions.[10]
-
Chunks of explosives-grade TNT
-
Trinitrotoluene melting at 81 °C (178 °F)
-
A group of M120 Rak mortar shells. The dark green shells on the left are stencilled to indicate a filling of TNT
-
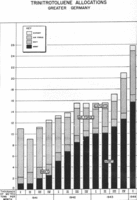
Analysis of TNT production by branch of the German armed forces between 1941 and the first quarter of 1944, shown in thousands of tons per month
-
Detonation of the 500-ton TNT explosive charge as part of Operation Sailor Hat in 1965. The passing blast-wave left a white water surface behind and a white condensation cloud is visible overhead.
Preparation[edit]
In industry, TNT is produced in a three-step process. First, toluene is nitrated with a mixture of sulfuric and nitric acid to produce mononitrotoluene (MNT). The MNT is separated and then renitrated to dinitrotoluene (DNT). In the final step, the DNT is nitrated to trinitrotoluene (TNT) using an anhydrous mixture of nitric acid and oleum. Nitric acid is consumed by the manufacturing process, but the diluted sulfuric acid can be reconcentrated and reused.
After nitration, TNT can either be purified by crystallization from an organic solvent or stabilized by a process called sulfitation, where the crude TNT is treated with aqueous sodium sulfite solution to remove less stable isomers of TNT and other undesired reaction products. The rinse water from sulfitation is known as red water and is a significant pollutant and waste product of TNT manufacture.[11]
Control of nitrogen oxides in feed nitric acid is very important because free nitrogen dioxide can result in oxidation of the methyl group of toluene. This reaction is highly exothermic and carries with it the risk of a runaway reaction leading to an explosion.[citation needed]
In the laboratory, 2,4,6-trinitrotoluene is produced by a two-step process. A nitrating mixture of concentrated nitric and sulfuric acids is used to nitrate toluene to a mixture of mono- and di-nitrotoluene isomers, with careful cooling to maintain temperature. The nitrated toluenes are then separated, washed with dilute sodium bicarbonate to remove oxides of nitrogen, and then carefully nitrated with a mixture of fuming nitric acid and sulfuric acid.[citation needed]
Applications[edit]
TNT is one of the most commonly used explosives for military, industrial, and mining applications. TNT has been used in conjunction with hydraulic fracturing (popularly known as fracking), a process used to recover oil and gas from shale formations. The technique involves displacing and detonating nitroglycerin in hydraulically induced fractures followed by wellbore shots using pelletized TNT.[12]
TNT is valued partly because of its insensitivity to shock and friction, with reduced risk of accidental detonation compared to more sensitive explosives such as nitroglycerin. TNT melts at 80 °C (176 °F), far below the temperature at which it will spontaneously detonate, allowing it to be poured or safely combined with other explosives. TNT neither absorbs nor dissolves in water, which allows it to be used effectively in wet environments. To detonate, TNT must be triggered by a pressure wave from a starter explosive, called an explosive booster.[13]
Although blocks of TNT are available in various sizes (e.g. 250 g, 500 g, 1,000 g), it is more commonly encountered in synergistic explosive blends comprising a variable percentage of TNT plus other ingredients. Examples of explosive blends containing TNT include:
- Amatex (ammonium nitrate and RDX)[14]
- Amatol (ammonium nitrate[15])
- Baratol (barium nitrate and wax[citation needed])
- Composition B (RDX and paraffin wax[16])
- Composition H6
- Cyclotol (RDX)[17]
- Ednatol
- Hexanite[citation needed] (hexanitrodiphenylamine[18][19])
- Minol
- Octol
- Pentolite
- Picratol
One Reply to “TNT #2”
Comments are closed.









PS3 Clan Theme