Buy now on iTunes for $0.99 http://itunes.apple.com/us/app/ninja-ambush/id409671385?mt=8
REALiZTiK_3
REALiZTiK_3 theme by S_Y_N_1_S_T_3_R
Download: REALiZTiK_3.p3t
P3T Unpacker v0.12
Copyright (c) 2007. Anoop Menon
This program unpacks Playstation 3 Theme files (.p3t) so that you can touch-up an existing theme to your likings or use a certain wallpaper from it (as many themes have multiple). But remember, if you use content from another theme and release it, be sure to give credit!
Download for Windows: p3textractor.zip
Instructions:
Download p3textractor.zip from above. Extract the files to a folder with a program such as WinZip or WinRAR. Now there are multiple ways to extract the theme.
The first way is to simply open the p3t file with p3textractor.exe. If you don’t know how to do this, right click the p3t file and select Open With. Alternatively, open the p3t file and it will ask you to select a program to open with. Click Browse and find p3textractor.exe from where you previously extracted it to. It will open CMD and extract the theme to extracted.[filename]. After that, all you need to do for any future p3t files is open them and it will extract.
The second way is very simple. Just drag the p3t file to p3textractor.exe. It will open CMD and extract the theme to extracted.[filename].
For the third way, first put the p3t file you want to extract into the same folder as p3textractor.exe. Open CMD and browse to the folder with p3extractor.exe. Enter the following:
p3textractor filename.p3t [destination path]Replace filename with the name of the p3t file, and replace [destination path] with the name of the folder you want the files to be extracted to. A destination path is not required. By default it will extract to extracted.filename.
Home Theater
Home Theater theme by Eman
Download: HomeTheater_1.p3t


A home cinema, also called a home theater or theater room, is a home entertainment audio-visual system that seeks to reproduce a movie theater experience and mood using consumer electronics-grade video and audio equipment and is set up in a room or backyard of a private home. Some studies show that films are rated better and generate more intense emotions when watched in a movie theater,[1] but convenience is a major appeal for home cinemas.[2] In the 1980s, home cinemas typically consisted of a movie pre-recorded on a LaserDisc or VHS tape; a LaserDisc Player or VCR; and a heavy, bulky large-screen cathode ray tube TV set, although sometimes CRT projectors were used instead. In the 2000s, technological innovations in sound systems, video player equipment and TV screens and video projectors have changed the equipment used in home cinema set-ups and enabled home users to experience a higher-resolution screen image, improved sound quality and components that offer users more options (e.g., many of the more expensive Blu-ray players in the 2020s can also stream movies and TV shows over the Internet using subscription services such as Netflix). The development of Internet-based subscription services means that 2020s-era home theatre users do not have to commute to a video rental store as was common in the 1980s and 1990s (nevertheless, some movie enthusiasts buy DVD or Blu-ray discs of their favorite content).
In the 2020s, a home cinema system typically uses a large projected image from a video projector or a large flat-screen high-resolution HDTV system, a movie or other video content on a DVD or high-resolution Blu-ray disc, which is played on a DVD player or Blu-ray player, with the audio augmented with a multi-channel power amplifier and anywhere from two speakers and a stereo power amp (for stereo sound) to a 5.1 channel amplifier and five or more surround sound speaker cabinets (with a surround sound system). Whether home cinema enthusiasts have a stereo set-up or a 5.1 channel surround system, they typically use at least one low-frequency subwoofer speaker cabinet to amplify low-frequency effects from movie soundtracks and reproduce the deep pitches from the musical soundtrack.
Introduction[edit]

Introduced in 1912, the Edison Home Projecting Kinetoscope was one of the first successful home theater devices. Although it used a 22 mm film format, the image size was nearer to 6 mm, the smallest ever to be commercially released. The value was that an entire motion picture could be released on one reel without editing.[3]

Home theater systems were made in the 1920s with 16 mm projectors. Technological improvements led to 8 mm and sound 16 mm in the 1930s. In the 1950s, playing home movies became popular in the United States with middle class and upper-class families as Kodak 8 mm film projector equipment became more affordable. The development of multi-channel audio systems and later LaserDisc in the 1980s created a new paradigm for home video, as it enabled movie enthusiasts to add better sound and images to their setup. In the mid-1980s to the mid-1990s, a typical home cinema in the United States would have a LaserDisc or VHS player playing a movie, with the signal fed to a large rear-projection television set, with the audio output through a stereo system. Some people used expensive front projectors in a darkened viewing room. During the 1980s, watching movies on VHS at home became a popular leisure activity. Beginning in the late 1990s, and continuing throughout much of the 2000s, home-theater technology progressed with the development of the DVD-Video format (higher resolution than VHS), Dolby Digital 5.1-channel audio (surround sound) speaker systems, and high-definition television (HDTV), which initially included bulky, heavy Cathode Ray Tube HDTVs and flat-screen TVs. In the 2010s, affordable large HDTV flatscreen TVs, high resolution video projectors (e.g., DLP), 3D television technology and the high resolution Blu-ray Disc (1080p) have ushered in a new era of home theater.
Recent developments[edit]
In the 2010s, the term home cinema encompasses a range of systems meant for movie playback at home. The most basic and economical system could be a DVD player, a standard-definition (SD) large-screen television with at least a 27-inch (69 cm) diagonal screen size, and an inexpensive home theater in a box surround sound amplifier and speaker system with a subwoofer. A more expensive home cinema set-up might include a Blu-ray disc player, home theater PC (HTPC) computer or digital media receiver streaming devices with a 10-foot user interface, a high-definition video projector and projection screen with over 100-inch (8.3 ft; 2.5 m) diagonal screen size (or a large flatscreen HDTV), and a several-hundred-watt home theater receiver with five to eleven surround-sound speakers plus one or two powerful subwoofers. 3D-TV-enabled home theaters make use of 3D TV sets/projectors and Blu-ray 3D players in which the viewers wear 3D-glasses, enabling them to see 3D content.
Home cinema designs and layouts are a personal choice and the type of home cinema a user can set up depends on her/his budget and the space which is available within the home. The minimum set of requirements for a home theater are: a large television set or good quality video projector CRT (no new models sold in U.S.), LCD, Digital Light Processing (DLP), plasma display, organic light-emitting diode (OLED), Silicon X-tal Reflective Display (SXRD), Laser TV, rear-projection TV, video projector, Standard-definition television (SDTV), HDTV, or 3D-TV at least 27 inches (69 cm) measured diagonally, an AV receiver or pre-amplifier (surround processor) and amplifier combination capable of at least stereo sound but preferably 5.1 Channel Dolby Digital and DTS audio, and something that plays or broadcasts movies in at least stereo sound such as a VHS HI-FI VCR, LaserDisc player (no new stand-alone models of either are available; VHS VCRs are usually bundled in combo decks with DVD players), a DVD player, a Blu-ray disc player, cable or satellite receiver, video game console, etc. Finally, a set of speakers, at least two, are needed but more common are anywhere from six to eight with a subwoofer for bass or low-frequency effects.[4]
The most expensive home cinema set-ups, which can cost over $100,000 US, and which are in the homes of executives, celebrities and high-earning professionals, have expensive, large, high-resolution digital projectors and projection screens, and maybe even custom-built screening rooms which include cinema-style chairs and audiophile-grade sound equipment designed to mimic (or sometimes even exceed) commercial cinema performance.
Design[edit]

In the 2010s, many home cinema enthusiasts aim to replicate, to the degree that is possible, the cinema experience. To do so, many home cinema buffs purchase higher quality components than used for everyday television viewing on a relatively small TV with only built-in speakers. A typical home cinema includes the following components:
- Movie or other viewing content: As the name implies, one of the key reasons for setting up a home cinema is to watch movies on a large screen, which does a more effective job at reproducing filmed images of vast landscapes or epic battle sequences. As of 2016, home cinema enthusiasts using smart Blu-ray players may also watch DVDs of TV shows, and recorded or live sports events or music concerts. As well, with a smart player, a user may be able to stream movies, TV shows and other content over the Internet. Many 2016-era DVD players and Blu-ray players also have inputs that allow users to view digital photos and other content on the big screen.
- Video and audio input devices: One or more video/audio sources. High-resolution movie media formats such as Blu-ray discs are normally preferred, though DVD or video game console systems are also used. Some home theaters include a HTPC (Home Theater PC) with a media center software application to act as the main library for video and music content using a 10-foot user interface and remote control. In 2016, some of the more expensive Blu-ray players can stream movies and TV shows over the Internet.
- Audio and video processing devices: Input signals are processed by either a standalone AV receiver or a preamplifier and Sound Processor for complex surround sound formats such as Dolby Pro-Logic/and or Pro-logic II, X, and Z, Dolby Digital, DTS, Dolby Digital EX, DTS-ES, Dolby Digital Plus, Dolby TrueHD, DTS-HD Master Audio, Dolby Atmos and DTS:X. The user selects the input (e.g., DVD, Blu-ray player, streaming video, etc.) at this point before it is forwarded to the output stage. Some AV receivers enable the viewer to use a remote control to select which input device or source to use.
- Audio output: Systems consist of preamplifiers, power amplifiers (both of which may be integrated into a single AV receiver) and two or more loudspeakers mounted in speaker enclosures. The audio system requires at least a stereo power amplifier and two speakers, for stereo sound; most systems have multi-channel surround sound power amplifier and six or more speakers (a 5.1 surround sound system has left and right front speakers, a centre speaker, left and right rear speakers and a low-frequency subwoofer speaker enclosure). Some users have 7.1 Surround Sound. It is possible to have up to 11 speakers with additional subwoofers.
- Video output: A large-screen display, typically an HDTV. Some users may have a 3D TV. As of 2015, flatscreen HDTVs are the norm. Options include Liquid crystal display television (LCD), plasma TV, and OLED.[5] Home cinema users may also use a video projector and a movie screen. If a projector is used, a portable, temporary screen may be used,a screen may be permanently mounted or the image may be projected directly on a wall.
- Seating and atmosphere: Comfortable seating is often provided to improve the cinema feel. Some luxury home cinemas have movie theatre-style padded chairs for guests. Higher-end home theaters commonly also have sound insulation to prevent noise from escaping the room and specialized wall treatment to balance the sound within the room. Wall color can be optimized.
Component systems vs. theater-in-a-box[edit]

Home cinemas can either be set up by purchasing individual components one by one (e.g., buying a multichannel amp from one manufacturer, a Blu-ray player from another manufacturer, speakers from another company, etc.) or a by purchasing a HTIB (Home Theater in a Box) package which includes all components from a single manufacturer, with the exception of a TV or projector. HTIB systems typically include a DVD or Blu-ray player, a surround sound amplifier, five surround speakers, a subwoofer cabinet, cables and a remote. The benefit of purchasing separate components one by one is that consumers can attain improved quality in video or audio and better matching between the components and the needs of a specific room, or the consumer\'s needs.
However, to buy individual components, a consumer must have knowledge of sound system and video system design and electronics and they must do research on the specifications of each component. For instance, some speakers perform better in smaller rooms while others perform better in larger rooms and seating location must be considered. One of the challenges with buying all the components separately is that the purchaser must understand speaker impedance, power handling, and HDMI compatibility and cabling. Given these challenges, HTIB systems are a simpler and more cost-effective solution for many families and consumers; they are also better suited to smaller living spaces in semi-detached homes or apartments/condos where noise could be an issue. Buying an HTIB package is often less expensive than buying separate components.
Dedicated rooms[edit]


Some home cinema enthusiasts build a dedicated room in their home for the theater. These more advanced installations often include sophisticated acoustic design elements, including room-in-a-room construction that isolates sound and provides an improved listening environment and a large screen, often using a high-definition projector. These installations are often designated as screening rooms to differentiate them from simpler, less-expensive installations. In some movie enthusiast\'s home cinemas, this idea can go as far as completely recreating an actual small-scale cinema, with a projector enclosed in its own projection booth, specialized furniture, curtains in front of the projection screen, movie posters, or a popcorn or vending machine with snack food and candy. More commonly, real dedicated home theaters pursue this to a lesser degree.
As of 2016, the days of the $100,000 and over home cinema system are being usurped by the rapid advances in digital audio and video technologies, which has spurred a rapid drop in prices, making a home cinema set-up more affordable than ever before. This in turn has brought the true digital home theater experience to the doorsteps of the do-it-yourselfers, often for much less than the price of a low-budget economy car. As of 2016, consumer-grade A/V equipment can meet some of the standards of a small modern commercial cinema (e.g., THX sound).
Seating[edit]
Home cinema seating consists of chairs or sofas specifically engineered and designed for viewing movies in a home cinema. Some home cinema seats have a cup holder built into the chairs\' armrests and a shared armrest between each seat. Some seating has cinema-style chairs like those seen in a cinema, which feature a flip-up seat cushion. Other seating systems have plush leather reclining lounger types, with flip-out footrests. Available features include storage compartments, snack trays, tactile transducers for low-frequency effects that can be felt through a chair (without creating high volume levels which could disturb other family members), and electric motors to adjust the chair. Home cinema seating tends to be more comfortable than seats in a public cinema.
Backyard cinema[edit]
In homes that have an adequately sized backyard, it is possible for people to set up a home cinema in an outdoor area. Depending on the space available, it may simply be a temporary version with foldable screen, a video projector and a couple of speakers, or a permanent fixture with a huge screen and dedicated audio set-up mounted in a weather-proof cabinet. Outdoor home cinemas are popular with BBQ parties and pool parties. Some specialist outdoor home-cinema companies are now marketing packages with inflatable movie screens and purpose-built AV systems. Some people have expanded the idea and constructed mobile drive-in theaters that can play movies in public open spaces. Usually, these require a powerful projector, a laptop or DVD player, outdoor speakers or an FM transmitter to broadcast the audio to other car radios.
History[edit]
1920s–1940s[edit]
In the 1920s the first home cinemas were made using silent 16mm film projectors such as Kodascope and Filmo. Later, in the 1930s, 8mm and sound 16mm were introduced. These were rare luxuries.
1950s–1970s[edit]
In the 1950s, home movies became more popular in the United States and elsewhere as Kodak 8 mm film (Pathé 9.5 mm in France) and camera and projector equipment became affordable. Projected with a small, portable movie projector onto a portable screen, often without sound, this system became the first practical home theater. They were generally used to show home movies of family travels and celebrations, but they also doubled as a means of showing some commercial films, or private stag films. Dedicated home cinemas were called screening rooms at the time and were outfitted with 16 mm or even 35 mm projectors for showing commercial films. These were found almost exclusively in the homes of the very wealthy, especially those in the movie industry.
Portable home cinemas improved over time with colour film, Kodak Super 8 mm film cartridges, and monaural sound but remained awkward and somewhat expensive. The rise of home video in the late 1970s almost completely killed the consumer market for 8 mm film cameras and projectors, as VCRs connected to ordinary televisions provided a simpler and more flexible substitute.
1980s[edit]
The development of multi-channel audio systems and LaserDisc in the 1980s added new dimensions for home cinema. The first-known home cinema system was designed, built and installed by Steve J. LaFontaine as a sales tool at Kirshmans furniture store in Metairie, Louisiana in 1974. He built a special sound room that incorporated the earliest quadraphonic audio systems, and he modified Sony Trinitron televisions for projecting the image. Many systems were sold in the New Orleans area in the ensuing years before the first public demonstration of this integration occurred in 1982 at the Summer Consumer Electronics Show in Chicago, Illinois. Peter Tribeman of NAD (U.S.) organized and presented a demonstration made possible by the collaborative effort of NAD, Proton, ADS, Lucasfilm and Dolby Labs, who contributed their technologies to demonstrate what a home cinema would look and sound like.
Over the course of three days, retailers, manufacturers, and members of the consumer electronics press were exposed to the first home-like experience of combining a high-quality video source with multi-channel surround sound. That one demonstration is credited with being the impetus for developing what is now a multibillion-dollar business.
1990s[edit]
In the early to mid-1990s, a typical home cinema would have a LaserDisc player or VHS VCR fed to a large screen: rear projection for the more-affordable setups, and LCD or CRT front-projection in the more-elaborate systems. In the late 1990s, a new wave of home-cinema interest was sparked by the development of DVD-Video, Dolby Digital and DTS 5.1-channel audio, and high-quality front video projectors that provide a cinema experience at a price that rivals a big-screen HDTV.
2000s[edit]

In the 2000s, developments such as high-definition video, Blu-ray disc (as well as the now-obsolete HD DVD format, which lost the format war to Blu-ray) and newer high-definition 3D display technologies enabled people to enjoy a cinematic feeling in their own home at a more-affordable price. Newer lossless audio from Dolby Digital Plus, Dolby TrueHD, DTS-HD High-Resolution Audio and DTS-HD Master Audio and speaker systems with more audio channels (such as 6.1, 7.1, 9.1, 9.2, 10.2, and 22.2) were also introduced for a more cinematic feeling.
2010s[edit]

By the mid-2010s, the Blu-ray Disc medium had become a common home media standard, and online video streaming sources such as Netflix, Hulu and YouTube were offering a range of high-definition content, including some 4K content (although various compression technologies are applied to make this streamed content feasible). The first 4K Blu-ray discs were released in 2016. By this point, 4K TVs and computer monitors were rapidly declining in price and increasing in prevalence, despite a lack of native 4K content. While many DSP systems existed, DTS-HD Master Audio remained the studio standard for lossless surround sound encoding on Blu-ray, with five or seven native discrete channels. High-definition video projectors also continued to improve and decrease in price, relative to performance.
As a result of continuing price reductions, large (up to 80\'\') TVs became a financially competitive alternative to video projectors in living room or even smaller dedicated room setups.[6] Technologies such as local dimming and the like improved the black levels of LCD screens making them more suitable for use in a dark room. Consumer-grade OLED TVs measuring 55\'\' and above began to emerge in the second half of the decade. These had even better black levels.[7] However, as of 2020, video projectors remained the only viable option when screen sizes much over 80\'\' are needed.
Entertainment equipment standards[edit]
Noise Criteria (NC) are noise-level guidelines applicable to cinema and home cinema. For this application, it is a measure of a room\'s ambient noise level at various frequencies. For example, in order for a theater to be THX certified, it must have an ambient sound level of NC-30 or less. This helps to retain the dynamic range of the system.[8] Some NC levels are:
- NC 40: Significant but not a dooming level of ambient noise; the highest acceptable ambient noise level. 40 decibels is the lower sound pressure level of normal talking; 60 being the highest.
- NC 30: A good NC level; necessary for THX certification in cinemas.
- NC 20: An excellent NC level; difficult to attain in large rooms and sought after for dedicated home cinema systems. For example, for a home cinema to be THX certified, it has to have a rating of NC 22.[9]
- NC 10: Virtually impossible noise criteria to attain; 10 decibels is associated with the sound level of calm breathing.
Projectors used for home cinemas have a set of recommended criteria:
- Brightness, usually at least 1800 lumens.
- Resolution (the number of pixels making up the image), usually at least 1920×1080, one of the HDTV standards.
- Contrast (how well white, black and greyscales are displayed), usually a minimum of 5000:1.[further explanation needed]
- HDMI connection sockets (although some people use older component video, connections with three-cord sockets for the different individual colours)
- Good quality manufacturers, although this is a subjective element that depends upon user tastes and budget. For one user with a modest budget, good quality may mean a mainstream consumer electronics brand; for a well-to-do user, a Christie projector may be their interpretation of good quality (Christie units are widely used in professional, commercial theatres)
See also[edit]
References[edit]
- ^ Fröber, K., & Thomaschke, R. (2019). In the dark cube: Movie theater context enhances the valuation and aesthetic experience of watching films. Psychology of Aesthetics, Creativity, and the Arts. Advance online publication.
- ^ Arnold, Andrew. "Convenience Vs. Experience: Millennials Love Streaming But Aren\'t Ready To Dump Cinema Just Yet". Forbes. Retrieved 21 May 2021.
- ^ "Edison\'s 22mm Home Kinetoscope". Hugh M. Hefner Moving Image Archive, University of Southern California. Retrieved 9 December 2023.
- ^ "How Much Cash Do I Need for a Home Theater Setup?". about.com. Retrieved 5 April 2018.
- ^ Feldstein, Justin.
I Love Music
I Love Music theme by mohnad
Download: ILoveMusic.p3t
I Love Music may refer to:
In music:
- "I Love Music" (The O'Jays song), a 1975 disco song written by Gamble and Huff and recorded by The O'Jays, covered in 1993 by Rozalla
- "I Love Music" (jazz composition), a jazz composition by Hale Smith with lyrics by Emil Boyd, first recorded in February 1970 by Ahmad Jamal.
In other areas:
- I Love Music (forum), an internet popular music forum founded in 2000
Microsoft Office 2010
Microsoft Office 2010 theme by Holland M.
Download: MicrosoftOffice2010.p3t
 s
s
Microsoft Office 2010 
 Microsoft Office 2010 applications from top left to bottom right: Word, Excel, PowerPoint and OneNote which collectively make up the Home and Student edition.
Microsoft Office 2010 applications from top left to bottom right: Word, Excel, PowerPoint and OneNote which collectively make up the Home and Student edition.Developer(s) Microsoft Initial release June 15, 2010[1] Final release Service Pack 2 (14.0.7268.5000) / April 15, 2021[2]Operating system Windows XP SP3 or later
Windows Server 2003 SP2 or later[3][4]Platform IA-32 and x64 Predecessor Microsoft Office 2007 (2007) Successor Microsoft Office 2013 (2013) Available in 40 languages[5] List of languagesEnglish, Arabic, Bulgarian, Chinese (Simplified), Chinese, Croatian, Czech, Danish, Dutch, Estonian, Filipino, Finnish, French, German, Greek, Hebrew, Hindi, Hungarian, Italian, Japanese, Kazakh, Korean, Latvian, Lithuanian, Norwegian (Bokmål), Polish, Portuguese (Brazil), Portuguese (Portugal), Romanian, Russian, Serbian, Slovak, Slovenian, Spanish, Swedish, Thai, Turkish, UkrainianType Office suite License Trialware Website products .office .com /office-2010 Microsoft Office 2010 (codenamed Office 14[6]) is a version of Microsoft Office for Microsoft Windows unveiled by Microsoft on May 15, 2009, and released to manufacturing on April 15, 2010,[1] with general availability on June 15, 2010.[7] The macOS equivalent, Microsoft Office 2011 for Mac was released on October 26, 2010.
Office 2010 introduces user interface enhancements including a Backstage view that consolidates document management tasks into a single location. The ribbon introduced in Office 2007 for Access, Excel, Outlook, PowerPoint, and Word is the primary user interface for all applications in Office 2010 and is now customizable.[8][9][10] Collaborative editing features that enable multiple users to share and edit documents;[11] extended file format support;[6] integration with OneDrive and SharePoint;[11] and security improvements such as Protected View, a sandbox to protect users from malicious content[12] are among its other new features. It debuted Office Online, free Web-based versions of Excel, OneNote, PowerPoint, and Word.[13][14][15] A new Office Starter 2010 edition replaces Microsoft Works.[16][17][18] Office Mobile 2010, an update to Microsoft's mobile productivity suite was released on May 12, 2010 as a free upgrade from the Windows Phone Store for Windows Mobile 6.5 devices with a previous version of Office Mobile installed.[19][20][21]
Office 2010 is the first version of Office to ship in a 64-bit version.[22][23] It is also the first version to require volume license product activation.[24][25] Office 2010 is compatible with Windows XP SP3 and Windows Server 2003 SP2 through Windows 10 v1809 and Windows Server 2016.[26][27] It is the last version of Microsoft Office to support Windows XP SP3, Windows Server 2003 SP2, Windows Vista SP1–SP2 and Windows Server 2008.[28][29][30][31]
Reviews of Office 2010 were generally very positive, with praise to the new Backstage view, new customization options for the ribbon, and the incorporation of the ribbon into all programs.[32][33] Sales, however, initially were lower than those of its predecessor.[34] Despite this, Office 2010 was a success for Microsoft, surpassing the company's previous records for adoption,[35] deployment,[35] and revenue for Office.[36] As of December 31, 2011, approximately 200 million licenses of Office 2010 were sold,[37] before its discontinuation on January 31, 2013.[38]
Mainstream support for Office 2010 ended on October 13, 2015, and extended support ended on October 13, 2020, the same dates that mainstream and extended support ended for Windows Embedded Standard 7.[39] Office 2010 is the last version of Office that can be activated without enrolling in a Microsoft account; enrollment for activation is required starting with Office 2013.[40] On June 9, 2018, Microsoft announced that its forums would no longer include Office 2010 or other products in extended support among its products for discussions involving support.[41] On August 27, 2021, Microsoft announced that Outlook 2010 and Outlook 2007 would be cut off from connecting to Microsoft 365 Exchange servers on November 1, 2021.[42]
History and development[edit]
Development started in 2007 while Microsoft was finishing work on Office 12, released as Microsoft Office 2007. The version number 13 was skipped because of the fear of the number 13.[43] It was previously thought that Office 2010 (then called Office 14) would ship in the first half of 2009.[44]
On April 15, 2009, Microsoft confirmed that Office 2010 would be released in the first half of 2010. They announced on May 12, 2009, at a Tech Ed event, a trial version of the 64-bit edition.[45][46] The Technical Preview 1 (Version: 14.0.4006.1010) was leaked on May 15, 2009.[47]
An internal post-beta build was leaked on July 12, 2009. This was newer than the official preview build and included a "Limestone" internal test application (note: the EULA indicates Beta 2).[48] On July 13, 2009, Microsoft announced Office 2010 at its Worldwide Partner Conference 2009.
On July 14, 2009, Microsoft started to send out invitations on Microsoft Connect to test an official preview build of Office 2010.[49] On August 30, 2009, the beta build 4417 was leaked on the internet via torrents.[50]
The public beta was available to subscribers of TechNet, MSDN and Microsoft Connect users on November 16, 2009.[51] On November 18, 2009, the beta was officially released to the general public at the Microsoft Office Beta website, which was originally launched by Microsoft on November 11, 2009 to provide screenshots of the new office suite.[52] Office 2010 Beta was a free, fully functional version and expired on October 31, 2010.[53]
In an effort to help customers and partners with deployment of Office 2010, Microsoft launched an Office 2010 application compatibility program with tools and guidance available for download.[54] On February 5, 2010, the official release candidate build 4734.1000 was available to Connect and MSDN testers. It was leaked to torrent sites.[55] A few days after, the RTM Escrow build was leaked.
Microsoft announced the RTM on April 15, 2010, and that the final version was to have speech technologies for use with text to speech in Microsoft OneNote, Microsoft PowerPoint, Microsoft Outlook, and Microsoft Word. Office 2010 was to be originally released to business customers on May 12, 2010,[56] however it was made available to Business customers with Software Assurance on April 27, 2010, and to other Volume Licensing Customers on May 1.[57] MSDN and TechNet subscribers have been able to download the RTM version since April 22, 2010. The RTM version number is 14.0.4763.1000.[58][59] Office 2010 was launched for general customer availability on June 15, 2010.[7][60]
Service packs[edit]
Service pack Version number Release date Service Pack 1 (SP1) 14.0.6029.1000[61] November 17, 2010[62] Service Pack 2 (SP2) 14.0.7015.1000[61] April 8, 2013[63] Microsoft released two service packs for Office 2010 that were primarily intended to address software bugs. Service Pack 1 (SP1) and Service Pack 2 (SP2) were released concurrently with updates for additional products including Office Online, SharePoint, and SharePoint Designer.[62][63]
On November 17, 2010, Microsoft invited a select number of testers at the Microsoft Connect Web portal to test SP1 Beta 1.[64][65] SP1 was released by Microsoft on June 27, 2011, and included compatibility, performance, security, and stability improvements. SP1 is a cumulative update that includes all previous updates, as well as fixes exclusive to its release;[62][66] a list of exclusive fixes was released by Microsoft.[67] SP1 also introduced additional features for Access, Excel, OneNote, Outlook, PowerPoint, and Word. As examples, OneNote 2010 SP1 introduced the ability to open notebooks stored in OneDrive directly from within the app itself, while Outlook 2010 SP1 introduced Microsoft 365 support.[66] With the release of SP1, the use of Office Online in Google Chrome and Internet Explorer 9 was officially supported by Microsoft for the first time.[68]
On April 8, 2013, a beta build of Office 2010 SP2 was released.[69] SP2 was a cumulative update officially released on July 16, 2013, and included all of the previously released compatibility, performance, stability, and security fixes, as well as numerous exclusive fixes;[63] a list of fixes exclusive to SP2 was released by Microsoft.[70] Microsoft claimed that with the release of SP2, Office 2010 would feature improved compatibility with Internet Explorer 10, Office 2013, and SharePoint 2013.[63][71] Because SP2 is cumulative, SP1 is not a prerequisite for its installation.[72]
New features[edit]
User interface[edit]
In both its client programs and in its Internet implementation, the design of Office 2010 incorporates features from SharePoint and borrows from Web 2.0 ideas.[73][74][75] Office 2010 is more "role-based" than previous versions of Microsoft Office, with specific features tailored to employees in "roles such as research and development professionals, sales people, and human resources."[75]
Backstage view[edit]
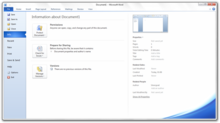
The Info tab in the navigation pane of Backstage displaying a document overview alongside management tasks in Word 2010. A new Backstage view interface replaces the Office menu introduced in Office 2007 and is designed to facilitate access to document management and sharing tasks by consolidating them within a single location.[76] In theatre, backstage refers to the area behind the stage where behind the scenes activities and preparations commence; the Backstage view is accordingly an interface dedicated to activities and preparations before saving or sharing a document.[77] Backstage consists of both a left-hand navigation pane and an adjacent main pane; the navigation pane includes a series of vertically arranged common commands to open or save files, and tabs that, when opened, expose document management tasks and contextual information within the main pane.[76][78] A customizable number of recently opened documents can also be displayed within the navigation pane.[79]
Tasks that are accessed via tabs in the main Backstage pane are categorized into separate groups that display contextual information related to app configurations, files, and tasks; each tab displays information relevant to that specific tab. On the Info tab in Word, for example, document metadata details are displayed within the Prepare for Sharing group to inform users of potentially personal information before the file is shared with other users,[80] whereas the Help tab displays Office 2010 version information and product licensing status.[81] In Office 2007, this information was included within separate locations.[80][81] From the Info tab, users can access revisions of currently open Excel, PowerPoint, and Word documents, as well as the latest unsaved version of a document that was previously closed.[82] Within the Print tab, Backstage also combines the previously separate print and print preview features by displaying printer tasks, settings, and a zooming user interface to preview the currently open document without the user having to open a dialog box.[83]
Backstage is extensible; developers can add their own commands, tabs, tasks, or related information.[84][85][86]
File tab[edit]
The File tab replaces the Office button introduced in Office 2007 and offers similar functionality. The previous Office button—a round button adorned with the Microsoft Office 2007 logo—had a different appearance from the ribbon tabs in the Office 2007 interface and was positioned away from them, with a target that extended toward the upper left corner of the screen in accordance with Fitts's law.[79][87] Microsoft stated this button enhanced the usability of Office, but many users saw it as "branding decoration, rather than a functional button." As a result, in Office 2010 it was replaced with a File tab that appears next to the other tabs in the ribbon instead of the upper left-hand corner of the screen.[79] The File tab is colored on a per-app basis (e.g., it is colored orange in Outlook). Opening the File tab displays the new Backstage view.[88]
Pasting options gallery[edit]
Office 2010 introduces a pasting options gallery on the ribbon, in the context menu, and in the object-oriented user interface that replaces the Paste Special dialog box and Paste Recovery feature seen in previous versions of Office. The gallery introduces Live Preview effects to the paste process when users position the mouse cursor over an option in the gallery so that the result of the process can be previewed before it is applied to the document; a tooltip with an associated description and keyboard shortcut for that option will also appear. If users position the mouse cursor over a gallery option in the context menu, the rest of the context menu becomes transparent so that it does not obstruct preview results within the document. To facilitate keyboard-based paste operations, users can navigate the gallery by using the arrow keys on a keyboard or press Ctrl after pressing Ctrl+V to display gallery options. Gallery options change based on the content in the clipboard and the app into which the content is pasted.[89]
Ribbon improvements[edit]
The ribbon introduced in Office 2007 is fully customizable and included in all programs in Office 2010.[8][9] Users can add or rename custom ribbon tabs or groups, add additional commands to the default tabs, and hide tabs that are not used. Users can also export or import any customization changes made to the ribbon to facilitate backups, deployment, or sharing, or reset all ribbon customizations.[90] The ribbon was also updated with a visible interface option to minimize it, which leaves only the tabs exposed.[91]
After the launch of Office 2010, Microsoft provided free downloads for a new Favorites tab that consolidated commands based on customer feedback regarding the most frequently used commands in all Office programs.[92]
Other UI changes[edit]
- The default color scheme in Office 2010 is silver instead of blue as in Office 2007 and now features a 5:1 contrast ratio to improve accessibility and readability.[88]
- All app icons have been redesigned in Office 2010. The new icons are based on colors that correspond to their respective programs, as per previous releases, with an increased emphasis on app letters.[88]
- The Office 2010 splash screen has been redesigned from the one seen in Office 2007 and animates when an app is launched.[88]
- OneNote and PowerPoint support mathematical equations through an Equation Tools contextual tab on the ribbon.[93][94]
- PowerPoint and Publisher include alignment guides so users can align objects to a grid.[94][95]
- Smart tags introduced in Office XP have been renamed as Actions and are now accessible from the context menu.[96]
File formats[edit]
Office 2010 includes updated support for ISO/IEC 29500, the International Standard version of Office Open XML (OOXML) file format.[6] Office 2010 provides read support for ECMA-376, read/write support for ISO/IEC 29500 Transitional, and read support for ISO/IEC 29500 Strict.[97] In its pre-release form, however, Office 2010 only supported the Transitional variant, and not the Strict.[98]
Office 2010 also continued support for OpenDocument Format (ODF) 1.1, which is a joint OASIS/ISO/IEC standard (ISO/IEC 26300:2006/Amd 1:2012 — Open Document Format for Office Applications (OpenDocument) v1.1).[6]
Document co-authoring[edit]
Office 2010 introduces co-authoring functionality in the Excel Web App, the OneNote Web App, and in the client versions of OneNote, PowerPoint, and Word for documents stored on SharePoint 2010 sites and for shared documents in OneDrive[11] and Microsoft 365.[99] A co-authoring session is automatically initiated when two or more users open the same document. From Backstage within Excel, OneNote, PowerPoint, and Word, users can also save documents directly to remote locations to facilitate remote access and co-authoring sessions. In the Excel Web App, the OneNote, and the OneNote Web App edits to a shared document in a co-authoring session occur on a sequential basis, in near real-time, as shared documents save automatically with each edit. In PowerPoint and Word, however, users must upload changes to the server by manually saving the shared document.[11]
During a co-authoring session the Excel Web App, PowerPoint, and Word denote how many co-authors are editing a document through a status bar icon that, when clicked in PowerPoint and Word, displays contact information including the presence of co-authors; the Info tab of Backstage also displays these details. When users open the name of a co-author, they can send email with an email client or start instant messaging conversations with each other if a supported app such as Skype for Business is installed on each machine. If a conflict between multiple changes occurs in PowerPoint or Word, sharers can approve or reject changes before uploading them to the server.[11]
In both OneNote and the OneNote Web App, users can view the names of co-authors alongside their respective edits to the content in a shared notebook, or create separate versions of pages for individual use. Edits made since a notebook was last opened are automatically highlighted, with initials of the co-author who made the edit displayed. In OneNote, co-authors can also search for all edits made by a specific co-author. OneNote 2010 notebooks can be shared with Office Mobile 2010 users on Windows Phone 7.[11] OneNote 2007 users can also participate in a co-authoring session with OneNote 2010 users if shared notebooks use the older OneNote 2007 file format; however, co-author search, and page versioning, and compatibility with the OneNote Web App will not be available.[100]
Installation and deployment[edit]
Office 2010 introduces a new Click-to-Run installation process based on Microsoft App-V Version 4 streaming and virtualization technology as an alternative to the traditional Windows Installer-based installation process for the Home and Student and Home and Business editions, and as a mandatory installation process for the Starter edition. Click-to-Run products install in a virtualized environment (a Q: partition) that downloads product features in the background after the programs have been installed so that users can immediately begin using the programs. The download process is optimized for broadband connections.[101]
During the Office 2010 retail lifecycle Microsoft, in collaboration with original equipment manufacturers (OEMs) and retail partners, introduced a Product Key Card licensing program that allowed users to purchase a single license to activate Home and Student, Home and Business, and Professional editions preinstalled on personal computers at a reduced cost when compared with traditional retail media.[102] Product Key Card versions are restricted to a single machine.[103]
Volume license versions of Office 2010 require product activation. Office 2007's product activation was only required for OEM or retail versions of the product.[24]
Security[edit]
Office File Validation[edit]
Office File Validation, previously included only in Publisher 2007 for PUB files has been incorporated into Excel, PowerPoint, and Word in Office 2010 to validate the integrity of proprietary binary file formats (e.g., DOC, PPT, and XLS) introduced in previous versions of Microsoft Office. When users open a document, the structure of its file format is scanned to ensure that it conforms with specifications defined by XML schema; if a file fails the validation process it will, by default, be opened in Protected View, a new read-only, isolated sandbox environment to protect users from potentially malicious content.[104] this design allows users to visually assess potentially unsafe documents that fail validation.[105] Microsoft stated that it is possible for documents to fail validation as a false positive. To improve Office File Validation, Office 2010 collects various information about files that have failed validation and also creates copies of these files for optional submission to Microsoft through Windows Error Reporting.[104] Users are prompted approximately every two weeks from the date of a failed validation attempt to submit copies of files or of other information for analysis; prompts include a list of files that will be submitted to Microsoft and require explicit user consent prior to submission. Administrators can disable data submission.[106]
On December 14, 2010, Microsoft announced it would backport Office File Validation to Office 2003 and Office 2007.[107][108] On April 12, 2011, it was backported as an add-in for Office 2003 SP3 and Office 2007 SP2, and on June 28, 2011, was made available through Microsoft Update.[109] Office File Validation in Office 2003 and Office 2007 differs from the version in Office 2010 as these two releases do not include the Protected View feature. When users attempt to open a document that fails validation, they must first agree to a warning prompt before it can be opened.[109] Additionally, the configuration options in these two releases are only made available through the Windows Registry,[110] whereas Office 2010 also provides Group Policy options.[104]
Protected View[edit]
Protected View, an isolated sandbox environment for Excel, PowerPoint, and Word, replaces the Isolated Conversion Environment update available for previous versions of Microsoft Office. When a document is opened from
Realistik
Realistik theme by Rews
Download: Realistik.p3t
P3T Unpacker v0.12
Copyright (c) 2007. Anoop MenonThis program unpacks Playstation 3 Theme files (.p3t) so that you can touch-up an existing theme to your likings or use a certain wallpaper from it (as many themes have multiple). But remember, if you use content from another theme and release it, be sure to give credit!
Download for Windows: p3textractor.zip
Instructions:
Download p3textractor.zip from above. Extract the files to a folder with a program such as WinZip or WinRAR. Now there are multiple ways to extract the theme.
The first way is to simply open the p3t file with p3textractor.exe. If you don’t know how to do this, right click the p3t file and select Open With. Alternatively, open the p3t file and it will ask you to select a program to open with. Click Browse and find p3textractor.exe from where you previously extracted it to. It will open CMD and extract the theme to extracted.[filename]. After that, all you need to do for any future p3t files is open them and it will extract.
The second way is very simple. Just drag the p3t file to p3textractor.exe. It will open CMD and extract the theme to extracted.[filename].
For the third way, first put the p3t file you want to extract into the same folder as p3textractor.exe. Open CMD and browse to the folder with p3extractor.exe. Enter the following:
p3textractor filename.p3t [destination path]Replace filename with the name of the p3t file, and replace [destination path] with the name of the folder you want the files to be extracted to. A destination path is not required. By default it will extract to extracted.filename.Recycled Jeans
Recycled Jeans theme by Holland M.
Download: RecycledJeans.p3t
P3T Unpacker v0.12
Copyright (c) 2007. Anoop MenonThis program unpacks Playstation 3 Theme files (.p3t) so that you can touch-up an existing theme to your likings or use a certain wallpaper from it (as many themes have multiple). But remember, if you use content from another theme and release it, be sure to give credit!
Download for Windows: p3textractor.zip
Instructions:
Download p3textractor.zip from above. Extract the files to a folder with a program such as WinZip or WinRAR. Now there are multiple ways to extract the theme.
The first way is to simply open the p3t file with p3textractor.exe. If you don’t know how to do this, right click the p3t file and select Open With. Alternatively, open the p3t file and it will ask you to select a program to open with. Click Browse and find p3textractor.exe from where you previously extracted it to. It will open CMD and extract the theme to extracted.[filename]. After that, all you need to do for any future p3t files is open them and it will extract.
The second way is very simple. Just drag the p3t file to p3textractor.exe. It will open CMD and extract the theme to extracted.[filename].
For the third way, first put the p3t file you want to extract into the same folder as p3textractor.exe. Open CMD and browse to the folder with p3extractor.exe. Enter the following:
p3textractor filename.p3t [destination path]Replace filename with the name of the p3t file, and replace [destination path] with the name of the folder you want the files to be extracted to. A destination path is not required. By default it will extract to extracted.filename.Porsche
Porsche theme by CatmClyde
Download: Porsche_3.p3t

Dr. Ing. h.c. F. Porsche AG 
 Headquarters in Stuttgart
Headquarters in StuttgartCompany type Public (AG) FWB: P911
DAX componentISIN DE000PAG9113 Industry Automotive Founded 1931 in Stuttgart, Germany Founder Ferdinand Porsche Headquarters Stuttgart, Germany Area servedWorldwide Key peopleWolfgang Porsche (chairman)
Oliver Blume (CEO)[1]Products Automobiles Production output 321,321 vehicles[2] (2022)
321,321 vehicles[2] (2022)Services Automotive financial services, engineering services, investment management Revenue  €37.630 billion (2022)[2]
€37.630 billion (2022)[2] €6.770 billion (2022)[2]
€6.770 billion (2022)[2] €4.957 billion (2022)[2]
€4.957 billion (2022)[2]Total assets  €47.673 billion (2022)[2]
€47.673 billion (2022)[2]Total equity  €17.027 billion (2022)[2]
€17.027 billion (2022)[2]Owners - Volkswagen AG (75%)
- Porsche SE (12.5%)
- QIA (2.5%)[3]
Number of employees39,162 (2022)[2] Subsidiaries - Mieschke Hofmann und Partner (81.8%)
- Porsche Consulting
- Bugatti Rimac (45%)
Website www .porsche .com Dr. Ing. h.c. F. Porsche AG, usually shortened to Porsche (German pronunciation: [ˈpɔʁʃə] ; see below), is a German automobile manufacturer specializing in luxury, high-performance sports cars, SUVs and sedans, headquartered in Stuttgart, Baden-Württemberg, Germany. The company is owned by Volkswagen AG, a controlling stake of which is owned by Porsche Automobil Holding SE. Porsche's current lineup includes the 718, 911, Panamera, Macan, Cayenne and Taycan.
The origins of the company date to the 1930s when Czech-German automotive engineer Ferdinand Porsche founded Porsche[4] with Adolf Rosenberger, a keystone figure in the creation of German automotive manufacturer and Audi precursor Auto Union,[5] and Austrian businessman Anton Piëch, who was, at the time, also Ferdinand Porsche's son in law. In its early days, it was contracted by the German government to create a vehicle for the masses, which later became the Volkswagen Beetle.[6] After World War II, when Ferdinand would be arrested for war crimes, his son Ferry Porsche began building his own car, which would result in the Porsche 356.
In 2009, Porsche entered an agreement with Volkswagen to create an 'integrated working group' by merging the two companies' car manufacturing operations.[7][8] By 2015, Porsche SE, the holding company spun off from the original Porsche firm, had a controlling interest in the Volkswagen Group, which included Audi and Lamborghini as subsidiaries.[9]
History[edit]
Origin[edit]
Ferdinand Porsche (1875–1951) founded the company called "Dr. Ing. h. c. F. Porsche GmbH"[4] with Adolf Rosenberger[10] and Anton Piëch in 1931.[11] The name is short for Ferdinand Porsche's full title in German, Doktor Ingenieur honoris causa lit. 'Doctor of Engineering, Honorary Degree' Ferdinand Porsche.[12] The main offices was at Kronenstraße 24 in the centre of Stuttgart.[13] Initially, the company offered motor vehicle development work and consulting,[4] but did not build any cars under its own name. One of the first assignments the new company received was from the German government to design a car for the people; that is, a Volkswagen.[4] This resulted in the Volkswagen Beetle, one of the most successful car designs of all time.[6] Later, the Porsche 64 would be developed in 1939 using many components from the Beetle.[4]

Porsche's tank prototype, the "Porsche Tiger", that lost to Henschel & Son's Tiger I 
Panzerjäger Elefant – after the loss of the contract to the Tiger I, Porsche recycled his design into a tank destroyer. During World War II,[14] Volkswagen production turned to the military version of the Volkswagen Beetle, the Kübelwagen,[14] 52,000 produced, and Schwimmwagen,[14] 15,584 produced.[15] Porsche produced several designs for heavy tanks during the war, losing out to Henschel & Son in both contracts that ultimately led to the Tiger I and the Tiger II. However, not all this work was wasted, as the chassis Porsche designed for the Tiger I was used as the base for the Elefant tank destroyer. Porsche also developed the Maus super-heavy tank in the closing stages of the war, producing two prototypes.[16] Ferdinand Porsche's biographer, Fabian Müller, wrote that Porsche had thousands of people forcibly brought to work at their factories during the war. The workers wore the letter "P" on their clothing at all times. It stood not for "Porsche", but for "Poland".[17]
At the end of World War II in 1945, the Volkswagen factory at KdF-Stadt fell to the British. Ferdinand lost his position as chairman of the board of management of Volkswagen, and Ivan Hirst, a British Army major, was put in charge of the factory. (In Wolfsburg, the Volkswagen company magazine dubbed him "The British Major who saved Volkswagen".)[18] On 15 December of that year, Ferdinand was arrested for war crimes, but not tried. During his 20-month imprisonment, Ferdinand Porsche's son, Ferry Porsche, decided to build his own car, because he could not find an existing one that he wanted to buy. He also had to steer the company through some of its most difficult days until his father's release in August 1947.[19]
The first models of what was to become the 356 were built in a small sawmill in Gmünd, Austria.[19] The prototype car was shown to German auto dealers, and when pre-orders reached a set threshold, production (with aluminum body) was begun by Porsche Konstruktionen GesmbH, founded by Ferry and Louise. Many regard the 356 as the first Porsche simply because it was the first model sold by the fledgling company. After production of the 356 was taken over by the father's Dr. Ing. h.c. F. Porsche GmbH in Stuttgart in 1950, Porsche commissioned a Zuffenhausen-based company, Reutter Karosserie, which had previously collaborated with the firm on Volkswagen Beetle prototypes, to produce the 356's steel body. In 1952, Porsche constructed an assembly plant (Werk 2) across the street from Reutter Karosserie; the main road in front of Werk 1, the oldest Porsche building, is now known as Porschestrasse.[20] The 356 was road-certified in 1948.
Company logo[edit]
Porsche's company logo stems from the coat of arms of the Free People's State of Württemberg of Weimar Germany of 1918–1933, which had Stuttgart as its capital. (The Bundesland of Württemberg-Hohenzollern used the same arms from 1945 to 1952, while Stuttgart during these years operated as the capital of adjacent Württemberg-Baden.) The arms of Stuttgart appear in the middle of the logo as an inescutcheon, for the company had its headquarters in Stuttgart. The heraldic symbols, combined with the texts "Porsche" and "Stuttgart", do not form a conventional coat of arms, since heraldic achievements never spell out the name of the armiger nor the armiger's home town in the shield.
Württemberg-Baden and Württemberg-Hohenzollern both in 1952 became part of the present Bundesland of Baden-Württemberg after the political consolidation of West Germany in 1949, but the old design of the arms of Württemberg lives on in the Porsche logo. On 30 January 1951, not long before the formation of Baden-Württemberg, Ferdinand Porsche died from complications following a stroke.
Developments[edit]

1952 Porsche 356 K/9-1 prototype In post-war Germany, parts were generally in short supply, so the 356 automobile used components from the Volkswagen Beetle, including the engine case from its internal combustion engine, transmission, and several parts used in the suspension. The 356, however, had several evolutionary stages, A, B, and C, while in production, and most Volkswagen-sourced parts were replaced by Porsche-made parts. Beginning in 1954 the 356s engines started utilizing engine cases designed specifically for the 356. The sleek bodywork was designed by Erwin Komenda, who also had designed the body of the Beetle. Porsche's signature designs have, from the beginning, featured air-cooled rear-engine configurations (like the Beetle), rare for other car manufacturers, but producing automobiles that are very well balanced.
In 1964, after a fair amount of success in motor-racing with various models including the 550 Spyder, and with the 356 needing a major re-design, the company launched the Porsche 911: another air-cooled, rear-engined sports car, this time with a six-cylinder "boxer" engine. The team to lay out the body shell design was led by Ferry Porsche's eldest son, Ferdinand Alexander Porsche (F. A.). The design phase for the 911 caused internal problems with Erwin Komenda, who led the body design department until then. F. A. Porsche complained Komenda made unauthorized changes to the design. Company leader Ferry Porsche took his son's drawings to neighbouring chassis manufacturer Reuter. Reuter's workshop was later acquired by Porsche (so-called Werk 2). Afterward, Reuter became a seat manufacturer, today known as Keiper-Recaro.

The Porsche 912, from the 1960s The design office gave sequential numbers to every project (See Porsche type numbers), but the designated 901 nomenclature contravened Peugeot's trademarks on all 'x0x' names, so it was adjusted to 911. Racing models adhered to the "correct" numbering sequence: 904, 906, 908. The 911 has become Porsche's most well-known model – successful on the race-track, in rallies, and in terms of road car sales. It remains in production; however, after several generations of revision, current-model 911s share only the basic mechanical configuration of a rear-engined, six-cylinder coupé, and basic styling cues with the original car. A cost-reduced model with the same body, but with a 356-derived four-cylinder engine, was sold as the 912.
In 1972, the company's legal form was changed from Kommanditgesellschaft (KG), or limited partnership, to Aktiengesellschaft (AG), or public limited company, because Ferry Porsche came to believe the scale of the company outgrew a "family operation", after learning about Soichiro Honda's "no family members in the company" policy at Honda. This led to the establishment of an executive board with members from outside the Porsche family, and a supervisory board consisting largely of family members. With this change, most family members in the operation of the company, including F. A. Porsche and Ferdinand Piëch, departed from the company.
F. A. Porsche founded his own design company, Porsche Design, which is renowned for exclusive sunglasses, watches, furniture, and many other luxury articles. Louise's son and Ferry's nephew Ferdinand Piëch, who was responsible for mechanical development of Porsche's production and racing cars (including the very successful 911, 908 and 917 models), formed his own engineering bureau, and developed a five-cylinder-inline diesel engine for Mercedes-Benz. A short time later he moved to Audi (used to be a division, then a subsidiary, of Volkswagen), and pursued his career through the entire company, ultimately becoming the chairman of Volkswagen Group.
The first chief executive officer (CEO) of Porsche AG was Ernst Fuhrmann, who had been working in the company's engine development division. Fuhrmann was responsible for the so-called Fuhrmann-engine, used in the 356 Carrera models as well as the 550 Spyder, having four overhead camshafts instead of a central camshaft with pushrods, as in the Volkswagen-derived serial engines. He planned to cease the 911 during the 1970s and replace it with the V8-front engined grand sportswagon 928. As we know today, the 911 outlived the 928 by far. Fuhrmann was replaced in the early 1980s by Peter W. Schutz, an American manager and self-proclaimed 911 aficionado. He was then replaced in 1988 by the former manager of German computer company Nixdorf Computer AG, Arno Bohn, who made some costly miscalculations that led to his dismissal soon after, along with that of the development director, Dr. Ulrich Bez, who was formerly responsible for BMW's Z1 model, and was CEO of Aston Martin from 2000 to 2013.[21]
Porsche 911 (964), introduced in 1989, was the first to be offered with Porsche's Tiptronic transmission and four-wheel drive. In 1990, Porsche drew up a memorandum of understanding with Toyota to learn and benefit from Japanese lean manufacturing methods. In 2004 it was reported that Toyota was assisting Porsche with hybrid technology.[22]
Following the dismissal of Bohn, Heinz Branitzki, a longtime Porsche employee, was appointed as interim CEO. Branitzki served in that position until Wendelin Wiedeking became CEO in 1993. Wiedeking took over the chairmanship of the board at a time when Porsche appeared vulnerable to a takeover by a larger company. During his long tenure, Wiedeking transformed Porsche into a very efficient and profitable company.
Ferdinand Porsche's nephew, Ferdinand Piëch, was chairman and CEO of the Volkswagen Group from 1993 to 2002 and is chairman of the Volkswagen AG Supervisory Board since then. With 12.8 percent of the Porsche SE voting shares, he also remains the second-largest individual shareholder of Porsche SE after his cousin, F. A. Porsche, which had 13.6 percent.
Porsche's 2002 introduction of the Cayenne also marked the unveiling of a new production facility in Leipzig, Saxony, which once accounted for nearly half of Porsche's annual output. In 2004, production of the 456 kilowatts (620 PS; 612 bhp) Carrera GT commenced in Leipzig, and at EUR 450,000 ($440,000 in the United States) it was the most expensive production model Porsche ever built.

Porsche 911 (991) In mid-2006, after years of the Boxster (and later the Cayenne) as the best selling Porsche in North America, the 911 regained its position as Porsche's best-seller in the region. The Cayenne and 911 have cycled as the top-selling model since. In Germany, the 911 outsells the Boxster/Cayman and Cayenne.[23]
In May 2011, Porsche Cars North America announced plans to spend $80–$100 million, but will receive about $15 million in economic incentives to move their North American headquarters from Sandy Springs, a suburb of Atlanta, to Aerotropolis, Atlanta, a new mixed-use development on the site of the old Ford Hapeville plant adjacent to Atlanta's airport.[24] Designed by architectural firm HOK, the headquarters will include a new office building and test track.[25][26][27] The facility will be known by its new address, One Porsche Drive.
In October 2017, Porsche Cars North America announced the launch of Porsche Passport,[28] a new sports car and SUV subscription program. This new offering allows consumers to access Porsche vehicles through subscribing to the service, rather than owning or leasing a vehicle. The Porsche Passport service was available initially in Atlanta,[29][30] and has become available in many major cities across the US.[31]
During the COVID-19 pandemic, in March 2020, Porsche suspended its manufacturing in Europe for two weeks, "By taking this step, the sports car manufacturer is responding to the significant acceleration in the rate of infection caused by the coronavirus and the resultant measures implemented by the relevant authorities."[32]
In August 2022, Bloomberg News reported that Porsche has lined up interest in subscription of its initial public offering for a valuation between US$60–85 billion. It is expected to be listed on Frankfurt Stock Exchange in September.[33]
Relationship with Volkswagen[edit]
Combined badging of the European 914 The company has always had a close relationship with, initially, the Volkswagen (VW) marque, and later, the Volkswagen Group (which also owns Audi AG), because the first Volkswagen Beetle was designed by Ferdinand Porsche.
The two companies collaborated in 1969 to make the VW-Porsche 914 and 914-6, whereby the 914-6 had a Porsche engine, and the 914 had a Volkswagen engine. Further collaboration in 1976 resulted in the Porsche 912E (US only) and the Porsche 924, which used many Audi components, and was built at Audi's Neckarsulm factory, which had been NSU's. Porsche 944s were also built there,[34] although they used far fewer Volkswagen components. The Cayenne, introduced in 2002, shares its chassis with the Volkswagen Touareg and the Audi Q7, which is built at the Volkswagen Group factory in Bratislava, Slovakia.
Corporate restructuring[edit]

Porsche Design Tower, Stuttgart 
A 991 in front of the factory in which it was assembled, Porsche-Werk Stuttgart (right), and the manufacturer's central dealership, Porsche Zentrum Stuttgart (left) Bleach
Bleach theme by SenamiPL
Download: Bleach_3.p3t


Clorox brand bleach Bleach is the generic name for any chemical product that is used industrially or domestically to remove colour (whitening) from fabric or fiber (in a process called bleaching) or to disinfect after cleaning. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
Many bleaches have broad-spectrum bactericidal properties, making them useful for disinfecting and sterilizing. They are used in swimming pool sanitation to control bacteria, viruses, and algae and in many places where sterile conditions are required. They are also used in many industrial processes, notably in the bleaching of wood pulp. Bleaches also have other minor uses, like removing mildew, killing weeds, and increasing the longevity of cut flowers.[1]
Bleaches work by reacting with many coloured organic compounds, such as natural pigments, and turning them into colourless ones. While most bleaches are oxidizing agents (chemicals that can remove electrons from other molecules), some are reducing agents (that donate electrons).
Chlorine, a powerful oxidizer, is the active agent in many household bleaches. Since pure chlorine is a toxic corrosive gas, these products usually contain hypochlorite, which releases chlorine. "Bleaching powder" usually refers to a formulation containing calcium hypochlorite.[citation needed]
Oxidizing bleaching agents that do not contain chlorine are usually based on peroxides, such as hydrogen peroxide, sodium percarbonate, and sodium perborate. These bleaches are called "non-chlorine bleach", "oxygen bleach", or "colour-safe bleach".[2]
Reducing bleaches have niche uses, such as sulfur dioxide, which is used to bleach wool, either as gas or from solutions of sodium dithionite,[3] and sodium borohydride.
Bleaches generally react with many other organic substances besides the intended coloured pigments, so they can weaken or damage natural materials like fibers, cloth, and leather, and intentionally applied dyes, such as the indigo of denim. For the same reason, ingestion of the products, breathing of the fumes, or contact with skin or eyes can cause bodily harm and damage health.
History[edit]

Early method of bleaching cotton and linen goods on lawns, using a combination of exposure to direct sunlight and the application of water The earliest form of bleaching involved spreading fabrics and cloth out in a bleachfield to be whitened by the action of the Sun and water.[4][5] In the 17th century, there was a significant cloth bleaching industry in Western Europe, using alternating alkaline baths (generally lye) and acid baths (such as lactic acid from sour milk, and later diluted sulfuric acid). The whole process lasted up to six months.[4]
Chlorine-based bleaches, which shortened that process from months to hours, were invented in Europe in the late 18th century. Swedish chemist Carl Wilhelm Scheele discovered chlorine in 1774,[4] and in 1785 Savoyard scientist Claude Berthollet recognized that it could be used to bleach fabrics.[4] Berthollet also discovered sodium hypochlorite, which became the first commercial bleach, named Eau de Javel ("Javel water") after the borough of Javel, near Paris, where it was produced.
Scottish chemist and industrialist Charles Tennant proposed in 1798 a solution of calcium hypochlorite as an alternative for Javel water, and patented bleaching powder (solid calcium hypochlorite) in 1799.[4][6] Around 1820, French chemist Antoine Germain Labarraque discovered the disinfecting and deodorizing ability of hypochlorites and was instrumental in popularizing their use for such purpose.[7] His work greatly improved medical practice, public health, and the sanitary conditions in hospitals, slaughterhouses, and all industries dealing with animal products.[8]
Louis Jacques Thénard first produced hydrogen peroxide in 1818 by reacting barium peroxide with nitric acid.[9] Hydrogen peroxide was first used for bleaching in 1882, but did not become commercially important until after 1930.[10] Sodium perborate as a laundry bleach has been used in Europe since the early twentieth century, and became popular in North America in the 1980s.[11]
Mechanism of action[edit]
Whitening[edit]
Colours of natural organic materials typically arise from organic pigments, such as beta carotene. Chemical bleaches work in one of two ways:
- An oxidizing bleach works by breaking the chemical bonds that make up the chromophore. This changes the molecule into a different substance that either does not contain a chromophore or contains a chromophore that does not absorb visible light. This is the mechanism of bleaches based on chlorine but also of oxygen-anions which react through the initial nucleophilic attack.[12]
- A reducing bleach works by converting double bonds in the chromophore into single bonds. This eliminates the ability of the chromophore to absorb visible light. This is the mechanism of bleaches based on sulfur dioxide.[13]
Sunlight acts as a bleach through a process leading to similar results: high-energy photons of light, often in the violet or ultraviolet range, can disrupt the bonds in the chromophore, rendering the resulting substance colourless. Extended exposure often leads to massive discolouration usually reducing the colours to a white and typically very faded blue.[14]
Antimicrobial efficacy[edit]
The broad-spectrum effectiveness of most bleaches is due to their general chemical reactivity against organic compounds, rather than the selective inhibitory or toxic actions of antibiotics. They irreversibly denature or destroy many proteins, including all prions, making them extremely versatile disinfectants.
Hypochlorite bleaches in low concentration were also found to attack bacteria by interfering with heat shock proteins on their walls.[15] According to 2013 Home Hygiene and Health report,[16] using bleach, whether chlorine- or peroxide-based, significantly increases germicidal efficiency of laundry even at low temperatures (30-40 degrees Celsius), which makes it possible to eliminate viruses, bacteria, and fungi from a variety of clothing in a home setting.[17]
Types of bleaches[edit]
Most industrial and household bleaches belong to three broad classes:
- Chlorine-based bleaches, whose active agent is chlorine, usually from the decomposition of some chlorine compound like hypochlorite or chloramine.
- Peroxide-based bleaches, whose active agent is oxygen, almost always from the decomposition of a peroxide compound like hydrogen peroxide.
- Sulfur dioxide-based bleaches, whose active agent is sulfur dioxide, possibly from the decomposition of some oxosulfur anion.
Chlorine-based bleaches[edit]
Chlorine-based bleaches are found in many household "bleach" products, as well as in specialized products for hospitals, public health, water chlorination, and industrial processes.
The grade of chlorine-based bleaches is often expressed as percent active chlorine. One gram of 100% active chlorine bleach has the same bleaching power as one gram of elemental chlorine.
The most common chlorine-based bleaches are:
- Sodium hypochlorite (NaClO), usually as a 3–6% solution in water, usually called "liquid bleach" or just "bleach". Historically called "Javel water" (French: eau de Javel). It is used in many households to whiten laundry, disinfect hard surfaces in kitchens and bathrooms, treat water for drinking, and keep swimming pools free of infectious agents.
- Bleaching powder (formerly known as "chlorinated lime"), usually a mixture of calcium hypochlorite (Ca(ClO)
2), calcium hydroxide (slaked lime, Ca(OH)
2), and calcium chloride (CaCl
2) in variable amounts.[18] Sold as a white powder or in tablets, it is used in many of the same applications as sodium hypochlorite but is more stable and contains more available chlorine. - Chlorine gas (Cl
2). It is used as a disinfectant in water treatment, especially to make drinking water and in large public swimming pools. It was used extensively to bleach wood pulp, but this use has decreased significantly due to environmental concerns. - Chlorine dioxide (ClO
2). This unstable gas is generated in situ or stored as dilute aqueous solutions. It finds large-scale applications for the bleaching of wood pulp, fats and oils, cellulose, flour, textiles, beeswax, skin, and in a number of other industries.
Other examples of chlorine-based bleaches, used mostly as disinfectants, are monochloramine, halazone, and sodium dichloroisocyanurate.[19][failed verification]
Peroxide-based bleaches[edit]
Peroxide-based bleaches are characterized by the peroxide chemical group, namely two oxygen atoms connected by a single bond, (–O–O–). This bond is easily broken, giving rise to very reactive oxygen species, which are the active agents of bleach.
The main products in this class are:
- Hydrogen peroxide itself (H
2O
2). It is used, for example, to bleach wood pulp and hair or to prepare other bleaching agents like perborates, percarbonates, peracids, etc. - Sodium percarbonate (Na
2H
3CO
6), an adduct of hydrogen peroxide and sodium carbonate ("soda ash" or "washing soda", Na
2CO
3). Dissolved in water, it yields a solution of the two products, that combines the degreasing action of the carbonate with the bleaching action of the peroxide. - Sodium perborate (Na
2H
4B
2O
8). Dissolved in water it forms some hydrogen peroxide, but also the perborate anion (B(OOH)(OH)−
3) which can perform nucleophilic oxidation.[20] - Peracetic (peroxoacetic) acid (H
3CC(O)OOH). Generated in situ by some laundry detergents, and also marketed for use as industrial and agricultural disinfection and water treatment.[21] - Benzoyl peroxide ((C
6H
5COO)
2). It is used in topical medications for acne[19] and to bleach flour.[22] - Ozone (O
3). While not properly a peroxide, its mechanism of action is similar. It is used in the manufacture of paper products, especially newsprint and white Kraft paper.[23] - Potassium persulfate (K2 S2O8) and other persulfate salts. It, alongside ammonium and sodium persulfate, is common in hair-lightening products.[24]
- Permanganate salts such as Potassium permanganate (KMnO4).
In the food industry, other oxidizing products like bromates are used as flour bleaching and maturing agents.
Reducing bleaches[edit]
Sodium dithionite (also known as sodium hydrosulfite) is one of the most important reductive bleaching agents. It is a white crystalline powder with a weak sulfurous odor. It can be obtained by reacting sodium bisulfite with zinc
- 2 NaHSO3 + Zn → Na2S2O4 + Zn(OH)2
It is used as such in some industrial dyeing processes to eliminate excess dye, residual oxide, and unintended pigments and for bleaching wood pulp.
Reaction of sodium dithionite with formaldehyde produces Rongalite,
- Na2S2O4 + 2 CH2O + H2O → NaHOCH2SO3 + NaHOCH2SO2
which is used in bleaching wood pulp, cotton, wool, leather and clay.[25]
Photographic bleach[edit]
In Negative film processing, silver halide grains are associated with couplers which, on development, produce metallic silver and a coloured image. The silver is 'bleached' to a soluble form in a solution of ferric EDTA, which is then dissolved in 'fix', a solution of sodium or ammonium thiosulfate. The procedure is the same for paper processing except that the EDTA and thiosulfate are mixed in 'bleachfix'.
In Reversal processing, residual silver in the emulsion after the first development is reduced to a soluble silver salt using a chemical bleach, most commonly EDTA. A conventional fixer then dissolves the reduced silver but leaves the unexposed silver halide intact. This unexposed halide is then exposed to light or chemically treated so that a second development produces a positive image. In colour and chromogenic film, this also generates a dye image in proportion to the silver.
Photographic bleaches are also used in black-and-white photography to selectively reduce silver to reduce silver density in negatives or prints. In such cases, the bleach composition is typically an acid solution of potassium dichromate.
Environmental impact[edit]
A Risk Assessment Report (RAR) conducted by the European Union on sodium hypochlorite conducted under Regulation EEC 793/93 concluded that this substance is safe for the environment in all its current, normal uses.[26] This is due to its high reactivity and instability. The disappearance of hypochlorite is practically immediate in the natural aquatic environment, reaching in a short time concentration as low as 10−22 μg/L or less in all emission scenarios. In addition, it was found that while volatile chlorine species may be relevant in some indoor scenarios, they have a negligible impact in open environmental conditions. Further, the role of hypochlorite pollution is assumed as negligible in soils.
Industrial bleaching agents can be sources of concern. For example, the use of elemental chlorine in the bleaching of wood pulp produces organochlorines and persistent organic pollutants, including dioxins. According to an industry group, the use of chlorine dioxide in these processes has reduced the dioxin generation to under-detectable levels.[27] However, the respiratory risk from chlorine and highly toxic chlorinated byproducts still exists.
A European study conducted in 2008 indicated that sodium hypochlorite and organic chemicals (e.g., surfactants, fragrances) contained in several household cleaning products can react to generate chlorinated volatile organic compounds (VOCs).[28] These chlorinated compounds are emitted during cleaning applications, some of which are toxic and probable human carcinogens. The study showed that indoor air concentrations significantly increase (8–52 times for chloroform and 1–1170 times for carbon tetrachloride, respectively, above baseline quantities in the household) during the use of bleach-containing products. The increase in chlorinated volatile organic compound concentrations was the lowest for plain bleach and the highest for the products in the form of "thick liquid and gel".
The significant increases observed in indoor air concentrations of several chlorinated VOCs (especially carbon tetrachloride and chloroform) indicate that bleach use may be a source that could be important in terms of inhalation exposure to these compounds. While the authors suggested that using these cleaning products may significantly increase the cancer risk,[28][29] this conclusion appears to be hypothetical:
- The highest level cited for a concentration of carbon tetrachloride (seemingly of highest concern) is 459 micrograms per cubic meter, translating to 0.073 ppm (part per million), or 73 ppb (part per billion). The OSHA-allowable time-weighted average concentration over eight hours is 10 ppm,[30] almost 140 times higher;
- The OSHA highest allowable peak concentration (5-minute exposure for five minutes in 4 hours) is 200 ppm,[30] twice as high as the reported highest peak level (from the headspace of a bottle of a sample of bleach plus detergent).
Disinfection[edit]
Sodium hypochlorite solution, 3–6%, (common household bleach) is typically diluted for safe use when disinfecting surfaces and when used to treat drinking water.[31][32]
A weak solution of 2% household bleach in warm water is typical for sanitizing smooth surfaces before the brewing of beer or wine.[citation needed]
US government regulations (21 CFR 178 Subpart C) allow food processing equipment and food contact surfaces to be sanitized with solutions containing bleach, provided that the solution is allowed to drain adequately before contact with food and that the solutions do not exceed 200 parts per million (ppm) available chlorine (for example, one tablespoon of typical household bleach containing 5.25% sodium hypochlorite, per gallon of water).
A 1-in-47 dilution of household bleach with water (1 part bleach to 47 parts water: e.g. one teaspoon of bleach in a cup of water, or 21 ml per litre, or 1/3 cup of bleach in a gallon of water) is effective against many bacteria and some viruses in homes.[33] Even "scientific-grade", commercially produced disinfection solutions such as Virocidin-X usually have sodium hypochlorite as their sole active ingredient, though they also contain surfactants (to prevent beading) and fragrances (to conceal the bleach smell).[34]
See hypochlorous acid for a discussion of the mechanism for disinfectant action.
An oral rinse with a 0.05% dilute solution of household bleach is shown to treat gingivitis.[35]
Colour safe bleach[edit]
Colour-safe bleach is a chemical that uses hydrogen peroxide as the active ingredient (for stain removal) rather than sodium hypochlorite or chlorine.[36] It also has chemicals in it that help brighten colours.[37] Though hydrogen peroxide is used for sterilization purposes and water treatment, its ability to disinfect laundry is limited because the concentration of hydrogen peroxide in laundry products is lower than what is used in other applications.[37]
Health hazards[edit]
The safety of bleaches depends on the compounds present, and their concentration.[38] Generally speaking, the ingestion of bleaches will cause damage to the esophagus and stomach, possibly leading to death. On contact with the skin or eyes, it causes irritation, drying, and potentially burns. Inhalation of bleach fumes can cause mild irritation of the upper airways..[38] Personal protective equipment should always be used when using bleach.
Bleach should never be mixed with vinegar or other acids, as this will create highly toxic chlorine gas, which can cause severe burns internally and externally.[39][40][41][42] Mixing bleach with ammonia similarly produces toxic chloramine gas, which can burn the lungs.[39][40][42] Mixing bleach with rubbing alcohol makes highly toxic chloroform,[43] while mixing with hydrogen peroxide results in an exothermic and potentially explosive chemical reaction that releases oxygen.[44]
False claims as a cure[edit]
 This article appears to be slanted towards recent events. (June 2024)
This article appears to be slanted towards recent events. (June 2024)Miracle Mineral Supplement (MMS), also promoted as "Master Mineral Solution" or "Chlorine Dioxide Solution" or CDS,[45] to evade restrictions by online retail platforms, is a bleach solution that has been fraudulently promoted as a cure-all since 2006.[46] Its main active ingredient is sodium chlorite, which is "activated" with citric acid to form chlorine dioxide. In an attempt to evade health regulations, its inventor Jim Humble, a former Scientologist, founded the Genesis II Church of Health and Healing, which considers MMS as its sacrament.[47][48]
During the COVID-19 pandemic, advocates of MMS including QAnon proponent Jordan Sather and Mark Grenon, who are affiliated with the Genesis II Church, began to suggest this would treat COVID-19.[49][50] Several news outlets, including The New York Times and The Washington Post, portrayed U.S. president Donald Trump's remarks in a 23 April 2020 briefing as promoting the injection of bleach as a potential COVID-19 treatment.[51][52][53] According to fact-checkers, many such reports were false and misleading, as they lacked needed context—including the fact that, at the same briefing, Trump specifically clarified that any such treatments "wouldn't be through injections, we're talking about almost a cleaning and sterilization of an area. Maybe it works, maybe it doesn't work, but it certainly has a big effect if it's on a stationary object.".[54][55] Nevertheless, the widespread reporting led the CDC, scientists, and bleach companies to re-state that bleach is harmful to humans and should not be ingested or injected.[56][53] MSN News quoted Professor Rob Chilcott, a
14 Destinations
14 Destinations theme by Jector
Download: 14Destinations.p3t
P3T Unpacker v0.12
Copyright (c) 2007. Anoop MenonThis program unpacks Playstation 3 Theme files (.p3t) so that you can touch-up an existing theme to your likings or use a certain wallpaper from it (as many themes have multiple). But remember, if you use content from another theme and release it, be sure to give credit!
Download for Windows: p3textractor.zip
Instructions:
Download p3textractor.zip from above. Extract the files to a folder with a program such as WinZip or WinRAR. Now there are multiple ways to extract the theme.
The first way is to simply open the p3t file with p3textractor.exe. If you don’t know how to do this, right click the p3t file and select Open With. Alternatively, open the p3t file and it will ask you to select a program to open with. Click Browse and find p3textractor.exe from where you previously extracted it to. It will open CMD and extract the theme to extracted.[filename]. After that, all you need to do for any future p3t files is open them and it will extract.
The second way is very simple. Just drag the p3t file to p3textractor.exe. It will open CMD and extract the theme to extracted.[filename].
For the third way, first put the p3t file you want to extract into the same folder as p3textractor.exe. Open CMD and browse to the folder with p3extractor.exe. Enter the following:
p3textractor filename.p3t [destination path]Replace filename with the name of the p3t file, and replace [destination path] with the name of the folder you want the files to be extracted to. A destination path is not required. By default it will extract to extracted.filename.


















